Abstract
INTRODUCTION:
Venous thromboembolism (VTE) is an important complication of many cancers. Although more widely recognized in solid tumors, VTE is still an appreciable cause of morbidity and mortality among patients with hematologic malignancies. For example, acute promyelocytic leukemia (APL) is a well-recognized risk factor for disseminated intravascular coagulation (DIC), which can lead to thrombosis. Patients with non-APL AML are also at increased risk for VTE, though the exact mechanisms are currently unclear. Thus, we sought to explore how patient demographics, comorbidities, hematologic factors, coagulation parameters, and cytogenetic and molecular profiles impact the incidence of VTE in patients with non-APL AML.
METHODS:
This was a retrospective observational case-control study of 120 non-APL AML patients treated at the University of Wisconsin-Madison. The electronic health record was manually reviewed for extraction of the relevant clinical information. Coagulation parameters assessed included INR, PTT, fibrinogen, D-dimer, and fibrin monomer. Coagulation data were collected both at diagnosis and during the first 7 days of induction, if applicable. Cytogenetics was categorized based on the presence of a normal karyotype or not. Genomic data were subdivided into eight different functional categories of genes that are commonly mutated in AML. Other factors that could potentially increase the risk of thrombosis were also analyzed, including age, sex, BMI, baseline blood counts, baseline bone marrow blast percentage, previous history of VTE, use of antifibrinolytic agents, and whether blood products were transfused during the early treatment period. The variables of interest between VTE cases and non-VTE cases were compared using the nonparametric Wilcoxon rank sum test.
RESULTS:
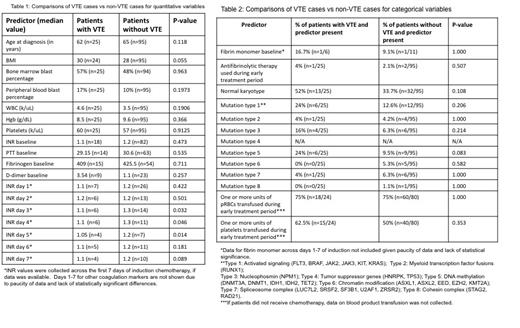
Across the entire cohort (n=120), VTE developed in 25 patients (20.8%) and did not develop in 95 patients (79.2%). Table 1 and Table 2 show comparisons between VTE cases and patients without VTE for both quantitative and categorical variables. Median INR values at diagnosis and across the first 7 days of induction tended to be lower in patients with VTE and reached significance on days 3-5 (p=0.032 for INR-Day 3, p=0.046 for INR-Day 4, p=0.014 for INR-Day 5). Patients with VTE had a median BMI of 30, while patients without VTE had a median BMI of 28, which approached statistical significance (p=0.055). 8% of patients with VTE (2/25) had a history of VTE prior to the diagnosis of AML, while 3% without VTE (3/95) had a history of previous VTE (OR 2.7, p=0.281). Mutation in genes involved in DNA methylation also conferred an increased risk of VTE, with the association approaching statistical significance (p=0.083). There were no other variables that were significantly different between the two groups.
CONCLUSIONS:
In this cohort of patients with non-APL AML, there was a trend towards lower INR correlating with an increased incidence of VTE. Additionally, higher BMI, previous history of VTE, and mutation in DNA methylation genes were associated with increased incidence of VTE, though these trends did not reach statistical significance. The pathophysiology of VTE in this setting is likely complex and multifactorial. The shorter INR in those who developed VTE suggests a mechanism independent of the development of DIC, which is distinct from APL. The finding that obesity and personal history of VTE, both known risk factors for thrombosis, were associated with an increased incidence of VTE is also consistent with a non-DIC mechanism. Mutations in DNA methylation genes may have contributed to increased risk of VTE in this cohort; while this association is compelling, further studies with larger sample sizes are needed to confirm this finding. This study was limited by small sample size and missing data, especially regarding available data on coagulation tests. However, this analysis uncovers interesting associations that shed light on the pathophysiology of VTE in AML. More research is needed to further elucidate risk factors for VTE in order to provide optimal supportive care for this patient population.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal